Abstract
Hematopoietic cell transplantation (HCT) is curative for many malignancies and genetic disorders but is hampered by significant early morbidity related to infection, regimen-related toxicities, acute graft versus host disease (GVHD), and risk of relapse. An excess risk of early death may be predicted by biomarkers associated with tissue healing, inflammation, and angiogenesis, as complementary to clinical variables. We have previously shown that pre-transplant low levels of one such biomarker, epidermal growth factor (EGF), is associated with poor survival after HCT. We have also shown that another EGF receptor ligand, amphiregulin (AREG), is elevated at the onset of aGVHD and is associated with a poor prognosis, especially if markedly elevated relative to EGF. AREG is active in type 2 immune responses and in dampening localized inflammation, but it found in the circulation in the states of unresolved tissue damage. We hypothesized that an elevated AREG relative to EGF prior to HCT would be associated with poor survival.
To test this hypothesis, we measured pre-HCT plasma EGF and AREG in 142 pediatric and adult HCT recipients from 2015-2017, with patients undergoing HCT for both malignant and non-malignant disease using various stem cell sources, GVHD prophylaxis, and conditioning regimens. We measured baseline plasma concentration of factors associated with tissue damage and repair, AREG by ELISA and EGF, vascular endothelial growth factor-A (VEGF-A), and placental growth factor (PlGF) by multiplex. We determined the optimal cutpoint for dichotomized analyses of AREG/EGF based on overall survival (OS) through 12 months post-transplant by method of Contal and O'Quigley, choosing a point that maximizes the log-rank statistic.
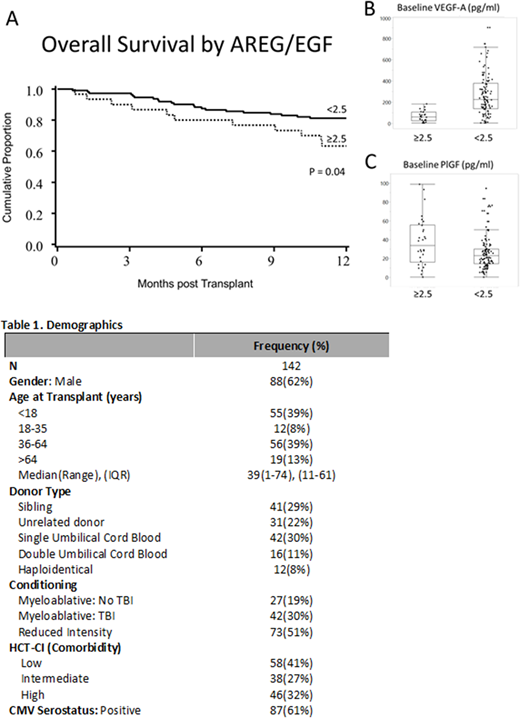
Patients (table) had a median 2.1 years of follow-up and a median baseline AREG/EGF ratio of 0.3 (IQR 0.1 - 1.7). A high baseline AREG/EGF ratio (cutoff of ≥2.5, in 30 patients, 21%) was associated with a >2-fold increased risk of all-cause mortality after HCT (HR 2.1, p = 0.037, Figure 1A). Clinical factors such as age, donor type, conditioning, disease, and overall comorbidity were not related to baseline AREG/EGF ratio. However, more patients with a baseline high AREG /EGF ratio had infections requiring systemic treatment at the time of transplant (26.7% vs 11.6%, p=0.039), higher rates of recent C. difficileinfections (23.3% vs 9.7%, p=0.046), suggesting a vulnerability to dysbiosis. Furthermore, a high AREG/EGF ratio at baseline was associated with other plasma biomarkers associated with tissue injury, with lower VEGF-A levels (61 vs 226 pg/ml, p<0.001, Figure 1B) and higher PlGF levels (34 vs 23 pg/ml, p=0.01, Figure 1C) prior to transplantation.
In this large and broad cohort of allograft recipients, over 20% of pediatric and adult HCT recipients have an elevated plasma AREG/EGF ratio prior to transplantation. These patients have a biomarker profile of unresolved tissue injury (low VEGF-A and high PlGF) and more frequent infections at the time of transplantation, including C. difficile. Further research into the systemic effects of dysbiosis may clarify the pathophysiology of this elevated AREG/EGF ratio and unresolved tissue injury. If confirmed, baseline AREG/EGF could identify patients most likely to benefit from novel tissue and/or microbial restorative therapies prior to HCT.
MacMillan:Equillium: Consultancy; CSL Behring: Consultancy; Angiocrine: Membership on an entity's Board of Directors or advisory committees. Weisdorf:Equillium: Consultancy; FATE: Consultancy; Seattle Genetics: Consultancy; SL Behring: Consultancy; Pharmacyclics: Consultancy. Holtan:Incyte: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.